RXBio Translates Sequence to Science and Industry
Tel: 027-87050299Email: sales@rxbio.cc
- Home
- Single-cell Sequencing
- Spatial Transcriptomics
- Third-generation Sequencing
- Omics Technologies
- Bioinformatics
- Experimental Platform
- About US
中文
RXBio Translates Sequence to Science and Industry
The label-free quantification (LFQ) technique does not require the use of expensive stable isotope tags as internal standards. It only needs to analyze the mass spectrometry data generated during the large-scale identification of proteins and compare the signal intensities of corresponding peptides in different samples, so as to perform relative quantification on the proteins corresponding to the peptides. It is typically applicable to the proteomics quantitative analysis of large cohorts of clinical blood/urine samples, subcellular components/exosomes, as well as the protein interactome analysis based on affinity purification.
✔ There is no limitation on the number of samples.
✔ Sample preparation is simple, and different samples can be processed separately.
✔ No labeling is required and the throughput is high.
-1024x227.jpg)
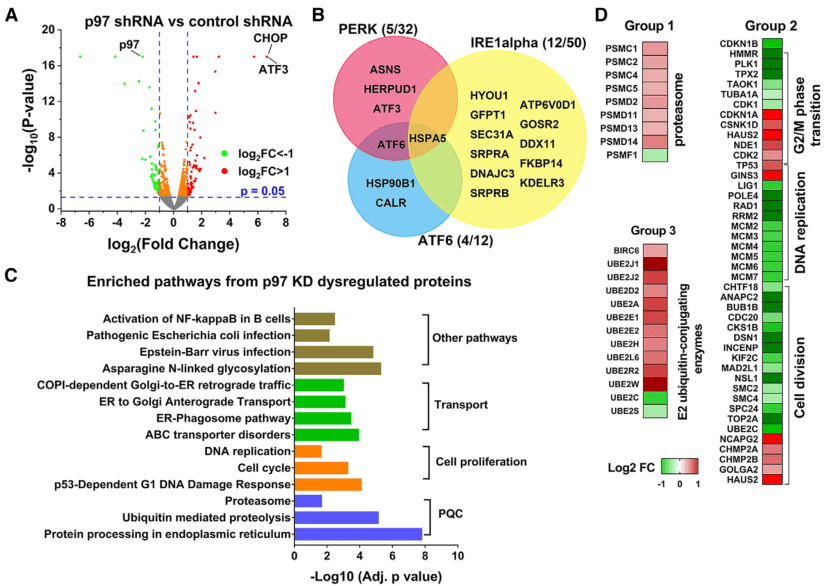
This study systematically compared the effects of p97 shRNA knockdown (KD) and three p97 inhibitors (CB-5083, NMS-873 and UPCDC-30245) with the proteasome inhibitor MG132 in HCT116 colon cancer cells by using proteomics analysis.
Label-free quantification (LFQ) proteomics analysis was employed to comprehensively measure the functional impact of p97 KD, and a total of 6,884 proteins were quantified. Compared with the control group, 410 significantly upregulated proteins and 356 significantly downregulated proteins (p-value < 0.05) were identified in p97 KD HCT116 cells. Differential analysis confirmed that the p97 protein was downregulated by 78%, and the ATF3 and DDIT3 (CHOP) proteins were upregulated more than 10-fold (Figure 1A). The changes in these proteins were consistent with the results of Western blotting (WB). Pathway enrichment analysis revealed that the differentially expressed proteins enriched in the three key unfolded protein response (UPR) pathways (PERK, ATF6 and IRE1α) were all upregulated, indicating that p97 deletion activated all three UPR pathways (Figure 1B). In addition, it was found that most cell cycle-related proteins were downregulated by p97 KD, including the MCM complex (Figure 1D). This finding is consistent with the lower proliferation rate of p97 KD cells and the important role of p97 in DNA replication.